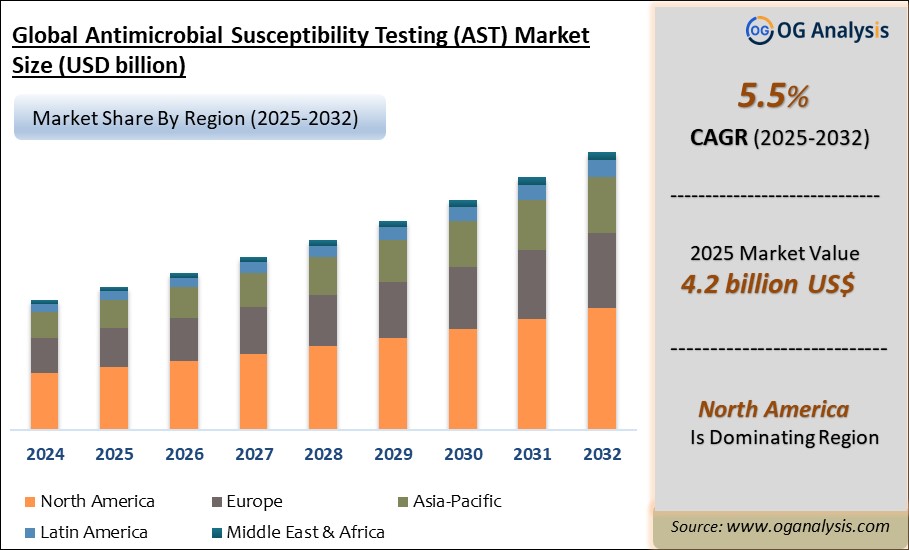
"The Global Antimicrobial Susceptibility Testing Market Size was valued at USD 4.0 billion in 2024 and is projected to reach USD 4.2 billion in 2025. Worldwide sales of Antimicrobial Susceptibility Testing are expected to grow at a significant CAGR of 5.5%, reaching USD 6.9 billion by the end of the forecast period in 2034."
The antimicrobial susceptibility testing (AST) market plays a vital role in modern healthcare systems, providing critical diagnostic tools to determine the effectiveness of antibiotics and guide targeted treatment decisions. This market has grown significantly in recent years due to the alarming rise of antimicrobial resistance (AMR) and the urgent need for robust surveillance and diagnostics to curb the misuse of antibiotics. AST methods, including manual, automated, and molecular-based systems, help healthcare professionals select the most appropriate antimicrobial therapy, reduce treatment failures, and limit the spread of resistant pathogens. Key end users include hospitals, diagnostic laboratories, research institutions, and pharmaceutical companies engaged in developing new antibiotics. Stringent regulatory requirements and increased awareness of hospital-acquired infections further drive market demand. Governments and global health bodies continue to emphasize better diagnostic stewardship, fueling investment in advanced and rapid AST solutions. The market benefits from a growing pipeline of novel AST devices that deliver faster, accurate results, minimizing the time needed to prescribe effective treatments.
Regionally, North America and Europe dominate the AST market, supported by strong healthcare infrastructure, high testing volumes, and proactive antimicrobial resistance management policies. However, Asia-Pacific is emerging as a high-growth region, driven by rising infection rates, expanding laboratory capacity, and increasing public and private healthcare investments. Automation and digitization trends are transforming laboratory workflows, enabling laboratories to handle larger testing volumes with higher accuracy and efficiency. Companies in the AST space are focusing on developing rapid, cost-effective, and point-of-care testing solutions to address the growing demand for quicker diagnostics in critical care settings. However, challenges such as high costs of advanced instruments, lack of skilled personnel in emerging markets, and regulatory hurdles can hamper wider adoption. Collaborations between industry players, research institutes, and government health agencies are crucial to accelerating new product approvals and expanding awareness about AMR. Overall, the antimicrobial susceptibility testing market is positioned for steady growth as it remains central to combating drug resistance and safeguarding global public health.
In the antimicrobial susceptibility testing market by product, consumables are the largest segment. This is because consumables such as media, reagents, and testing panels are required regularly for each test performed in laboratories and hospitals. Their recurring use generates consistent demand, making consumables the dominant revenue contributor for companies operating in the market.
By application, clinical diagnostics is the largest segment in the antimicrobial susceptibility testing market. This dominance is driven by the high burden of infectious diseases and hospital-acquired infections, requiring routine testing to guide appropriate antimicrobial therapy. Hospitals and diagnostic laboratories conduct a large volume of these tests, ensuring strong and stable demand for clinical diagnostic applications.
Key Insights
- Increasing prevalence of antimicrobial resistance globally is the primary driver for the AST market, as clinicians and hospitals rely on accurate testing to choose effective treatments. Rising incidences of multi-drug resistant infections are pushing healthcare providers to adopt advanced diagnostic tools for timely intervention and infection control.
- Automated antimicrobial susceptibility testing systems are witnessing higher adoption due to their ability to deliver standardized, high-throughput, and rapid results. Automation minimizes human error, reduces turnaround time, and enhances workflow efficiency in busy hospital and reference laboratories.
- The market is also benefiting from technological advancements such as rapid molecular diagnostic platforms and next-generation sequencing (NGS) tools. These solutions enable faster pathogen identification and resistance profiling, supporting precision medicine initiatives in infectious disease management.
- Hospitals and diagnostic laboratories are the largest end-users, driven by the high burden of hospital-acquired infections and the need for routine susceptibility testing. Increasing awareness of infection control practices and regulatory mandates for AMR surveillance are further boosting demand in these segments.
- Asia-Pacific is emerging as a high-potential market for AST, supported by growing investments in healthcare infrastructure, rising awareness of AMR, and government-led surveillance programs. Countries like India and China are scaling up laboratory capacity to handle rising infection rates and improve diagnostic accuracy.
- Point-of-care antimicrobial susceptibility testing is gaining traction, especially for use in emergency departments and outpatient clinics. The ability to provide rapid results at the patient’s bedside is transforming clinical decision-making and reducing unnecessary antibiotic prescriptions.
- Leading companies are investing in research and development to launch innovative testing panels, consumables, and integrated diagnostic platforms. Partnerships with research institutions and public health organizations are key strategies to enhance technology validation and market penetration.
- Stringent regulatory frameworks and quality standards ensure reliability of AST results but can pose challenges for new product approvals. Companies must navigate diverse country-specific regulations and obtain multiple certifications to market their products globally.
- Shortage of trained laboratory personnel, particularly in low- and middle-income countries, is a major constraint. This creates a need for user-friendly, automated systems that can be operated with minimal specialized training while maintaining accuracy and reliability.
- Collaborative efforts among healthcare providers, diagnostic manufacturers, and policymakers are vital to curb antimicrobial resistance. Expanding educational campaigns and funding support for diagnostic stewardship programs play an important role in driving adoption of AST technologies worldwide.
Reort Scope
| Parameter | Detail |
|---|---|
| Base Year | 2024 |
| Estimated Year | 2025 |
| Forecast Period | 2026-2034 |
| Market Size-Units | USD billion |
| Market Splits Covered | By Product, By Method, By Application, By End User |
| Countries Covered | North America (USA, Canada, Mexico) Europe (Germany, UK, France, Spain, Italy, Rest of Europe) Asia-Pacific (China, India, Japan, Australia, Rest of APAC) The Middle East and Africa (Middle East, Africa) South and Central America (Brazil, Argentina, Rest of SCA) |
| Analysis Covered | Latest Trends, Driving Factors, Challenges, Supply-Chain Analysis, Competitive Landscape, Company Strategies |
| Customization | 10 % free customization (up to 10 analyst hours) to modify segments, geographies, and companies analyzed |
| Post-Sale Support | 4 analyst hours, available up to 4 weeks |
| Delivery Format | The Latest Updated PDF and Excel Datafile |
Market Segmentation
By Product:
- Manual Testing Products
- Automated Testing Systems
- Consumables
By Method:
- Disk Diffusion
- Dilution
- E-test
By Application:
- Clinical Diagnostics
- Drug Discovery and Development
- Others
By End User:
- Hospitals and Diagnostic Centers
- Pharmaceutical and Biotechnology Companies
- Research and Academic Institutes
By Regian
- North America (USA, Canada, Mexico)
- Europe (Germany, UK, France, Spain, Italy, Rest of Europe)
- Asia-Pacific (China, India, Japan, Australia, Rest of APAC)
- The Middle East and Africa (Middle East, Africa)
- South and Central America (Brazil, Argentina, Rest of SCA)
What You Receive
• Global Antimicrobial Susceptibility Testing market size and growth projections (CAGR), 2024- 2034• Impact of recent changes in geopolitical, economic, and trade policies on the demand and supply chain of Antimicrobial Susceptibility Testing.
• Antimicrobial Susceptibility Testing market size, share, and outlook across 5 regions and 27 countries, 2025- 2034.
• Antimicrobial Susceptibility Testing market size, CAGR, and Market Share of key products, applications, and end-user verticals, 2025- 2034.
• Short and long-term Antimicrobial Susceptibility Testing market trends, drivers, restraints, and opportunities.
• Porter’s Five Forces analysis, Technological developments in the Antimicrobial Susceptibility Testing market, Antimicrobial Susceptibility Testing supply chain analysis.
• Antimicrobial Susceptibility Testing trade analysis, Antimicrobial Susceptibility Testing market price analysis, Antimicrobial Susceptibility Testing Value Chain Analysis.
• Profiles of 5 leading companies in the industry- overview, key strategies, financials, and products.
• Latest Antimicrobial Susceptibility Testing market news and developments.
The Antimicrobial Susceptibility Testing Market international scenario is well established in the report with separate chapters on North America Antimicrobial Susceptibility Testing Market, Europe Antimicrobial Susceptibility Testing Market, Asia-Pacific Antimicrobial Susceptibility Testing Market, Middle East and Africa Antimicrobial Susceptibility Testing Market, and South and Central America Antimicrobial Susceptibility Testing Markets. These sections further fragment the regional Antimicrobial Susceptibility Testing market by type, application, end-user, and country.
Who can benefit from this research
The research would help top management/strategy formulators/business/product development/sales managers and investors in this market in the following ways1. The report provides 2024 Antimicrobial Susceptibility Testing market sales data at the global, regional, and key country levels with a detailed outlook to 2034, allowing companies to calculate their market share and analyze prospects, uncover new markets, and plan market entry strategy.
2. The research includes the Antimicrobial Susceptibility Testing market split into different types and applications. This segmentation helps managers plan their products and budgets based on the future growth rates of each segment
3. The Antimicrobial Susceptibility Testing market study helps stakeholders understand the breadth and stance of the market giving them information on key drivers, restraints, challenges, and growth opportunities of the market and mitigating risks
4. This report would help top management understand competition better with a detailed SWOT analysis and key strategies of their competitors, and plan their position in the business
5. The study assists investors in analyzing Antimicrobial Susceptibility Testing business prospects by region, key countries, and top companies' information to channel their investments.
Available Customizations
The standard syndicate report is designed to serve the common interests of Antimicrobial Susceptibility Testing Market players across the value chain and include selective data and analysis from entire research findings as per the scope and price of the publication.However, to precisely match the specific research requirements of individual clients, we offer several customization options to include the data and analysis of interest in the final deliverable.
Some of the customization requests are as mentioned below :
Segmentation of choice – Our clients can seek customization to modify/add a market division for types/applications/end-uses/processes of their choice.
Antimicrobial Susceptibility Testing Pricing and Margins Across the Supply Chain, Antimicrobial Susceptibility Testing Price Analysis / International Trade Data / Import-Export Analysis
Supply Chain Analysis, Supply–Demand Gap Analysis, PESTLE Analysis, Macro-Economic Analysis, and other Antimicrobial Susceptibility Testing market analytics
Processing and manufacturing requirements, Patent Analysis, Technology Trends, and Product Innovations
Further, the client can seek customization to break down geographies as per their requirements for specific countries/country groups such as South East Asia, Central Asia, Emerging and Developing Asia, Western Europe, Eastern Europe, Benelux, Emerging and Developing Europe, Nordic countries, North Africa, Sub-Saharan Africa, Caribbean, The Middle East and North Africa (MENA), Gulf Cooperation Council (GCC) or any other.
Capital Requirements, Income Projections, Profit Forecasts, and other parameters to prepare a detailed project report to present to Banks/Investment Agencies.
Customization of up to 10% of the content can be done without any additional charges.
Note: Latest developments will be updated in the report and delivered within 2 to 3 working days.
Research Methodology
Our research methodology combines primary and secondary research techniques to ensure comprehensive market analysis.
Primary Research
We conduct extensive interviews with industry experts, key opinion leaders, and market participants to gather first-hand insights.
Secondary Research
Our team analyzes published reports, company websites, financial statements, and industry databases to validate our findings.
Data Analysis
We employ advanced analytical tools and statistical methods to process and interpret market data accurately.
Get Free Sample
At OG Analysis, we understand the importance of informed decision-making in today's dynamic business landscape. To help you experience the depth and quality of our market research reports, we offer complimentary samples tailored to your specific needs.
Start Now! Please fill the form below for your free sample.
Why Request a Free Sample?
Evaluate Our Expertise: Our reports are crafted by industry experts and seasoned analysts. Requesting a sample allows you to assess the depth of research and the caliber of insights we provide.
Tailored to Your Needs: Let us know your industry, market segment, or specific topic of interest. Our free samples are customized to ensure relevance to your business objectives.
Witness Actionable Insights: See firsthand how our reports go beyond data, offering actionable insights and strategic recommendations that can drive your business forward.
Embark on your journey towards strategic decision-making by requesting a free sample from OG Analysis. Experience the caliber of insights that can transform the way you approach your business challenges.
FAQ's
The Antimicrobial Susceptibility Testing Market is estimated to reach USD 6.1 billion by 2032.
The Global Antimicrobial Susceptibility Testing Market is expected to grow at a Compound Annual Growth Rate (CAGR) of 5.5% during the forecast period from 2025 to 2032.
The Global Antimicrobial Susceptibility Testing Market is estimated to generate USD 4 billion in revenue in 2024.
$4150- 30%
$6450- 40%
$8450- 50%
$2850- 20%
Didn’t find what you’re looking for? TALK TO OUR ANALYST TEAM
Need something within your budget? NO WORRIES! WE GOT YOU COVERED!

