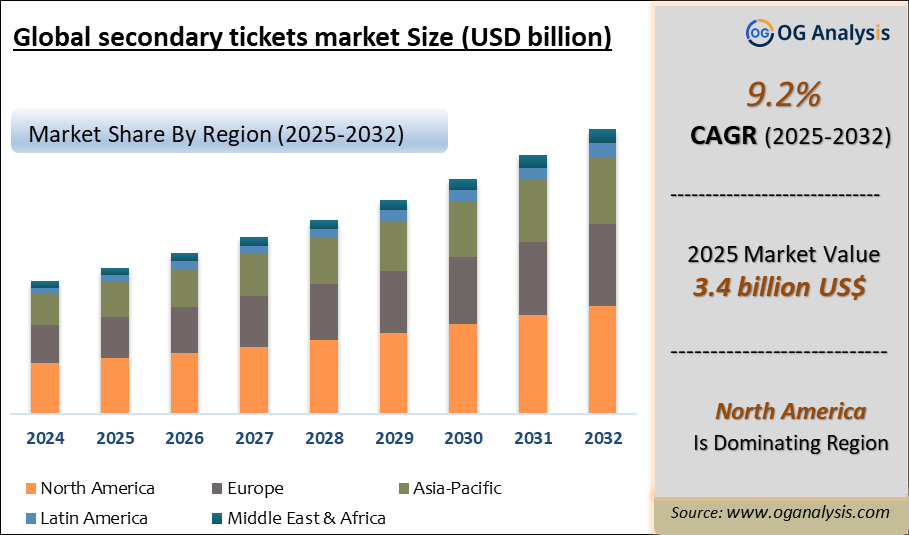
""The Global Secondary Tickets Market valued at USD 3.2 Billion in 2024, is expected to grow by 9.2% CAGR to reach market size worth USD 7.8 Billion by 2034.""
"The Global Secondary Tickets Market valued at USD 3.2 Billion in 2024, is expected to grow by 9.2% CAGR to reach market size worth USD 7.8 Billion by 2034."
The secondary ticket market is experiencing a profound transformation, driven by technological innovations, evolving consumer behaviors, and the ever-changing landscape of live entertainment. With the rise of digital platforms and the growing acceptance of resale ticketing, stakeholders in this market are witnessing unprecedented growth opportunities. The ongoing integration of artificial intelligence and blockchain technology is enhancing transparency and security, further appealing to consumers. As we head into 2025, the expected uptick in demand for event attendance, coupled with increased flexibility in purchasing and reselling tickets, positions the secondary ticket market for remarkable expansion.
In 2024, significant developments have reshaped the secondary ticket market landscape. Companies are increasingly leveraging data analytics to understand consumer preferences, allowing them to offer personalized experiences and targeted marketing strategies. This trend aligns with the heightened emphasis on customer engagement and satisfaction. Additionally, regulatory changes and partnerships with major event organizers are establishing a more structured environment for ticket resale, leading to improved trust and reliability among consumers. As event organizers explore new ways to monetize their offerings, the secondary ticket market is evolving from merely a resale platform to a vital component of the overall ticketing ecosystem, paving the way for enhanced consumer interactions and revenue streams.
The Global Secondary Tickets Market Analysis Report will provide a comprehensive assessment of business dynamics, offering detailed insights into how companies can navigate the evolving landscape to maximize their market potential through 2034. This analysis will be crucial for stakeholders aiming to align with the latest industry trends and capitalize on emerging market opportunities.
Secondary Tickets Market Strategy, Price Trends, Drivers, Challenges and Opportunities to 2034
In terms of market strategy, price trends, drivers, challenges, and opportunities from2025 to 2034, Secondary Tickets market players are directing investments toward acquiring new technologies, securing raw materials through efficient procurement and inventory management, enhancing product portfolios, and leveraging capabilities to sustain growth amidst challenging conditions. Regional-specific strategies are being emphasized due to highly varying economic and social challenges across countries.
Factors such as global economic slowdown, the impact of geopolitical tensions, delayed growth in specific regions, and the risks of stagflation necessitate a vigilant and forward-looking approach among Secondary Tickets industry players. Adaptations in supply chain dynamics and the growing emphasis on cleaner and sustainable practices further drive strategic shifts within companies.
The market study delivers a comprehensive overview of current trends and developments in the Secondary Tickets industry, complemented by detailed descriptive and prescriptive analyses for insights into the market landscape until 2034.
North America Secondary Tickets Market Analysis
The North American Secondary Tickets market experienced significant advancements in 2024, driven by heightened consumer focus on sustainability, technological integration, and personalized offerings across various segments. Growth was propelled by the rise in demand for innovative packaging solutions, eco-friendly products, and digital transformation in retail and service sectors. Companies leveraged advanced technologies such as AI, IoT, and data analytics to enhance customer engagement, optimize supply chains, and develop targeted marketing strategies. From 2025, the market is anticipated to witness robust expansion, underpinned by increasing adoption of subscription-based services, heightened awareness of eco-conscious consumption, and innovations in packaging and delivery methods. A competitive landscape characterized by continuous product differentiation, strategic mergers and acquisitions, and the influx of startups is reshaping market dynamics, with key players investing in digitalization and sustainability to secure market share.
Europe Secondary Tickets Market Outlook
The European Secondary Tickets market in 2024 demonstrated strong momentum, underpinned by regulatory emphasis on sustainable practices and consumer preferences for high-quality, eco-friendly, and customizable products. Rising interest in cultural and experiential offerings, coupled with advancements in e-commerce and digital solutions, bolstered market growth. The region's focus on circular economy principles encouraged investments in recyclable and biodegradable packaging solutions. Moving into 2025, growth is expected to be driven by the increasing prevalence of innovative retail models, AI-driven personalization, and a surge in demand for wellness-related consumer products. The competitive landscape is marked by robust participation from regional leaders and multinational firms, adopting strategies such as partnerships and green initiatives to meet regulatory and consumer demands, positioning Europe as a hub of innovation and sustainable growth.
Asia-Pacific Secondary Tickets Market Forecast
The Asia-Pacific Secondary Tickets market witnessed dynamic growth in 2024, fueled by rapid urbanization, digital adoption, and evolving consumer preferences for convenience and premium products. Emerging markets played a pivotal role, with increasing disposable income and a young, tech-savvy population driving demand for connected home devices, innovative retail solutions, and functional packaging. Anticipated growth from 2025 stems from an expanding middle class, escalating e-commerce penetration, and strong demand for personalized and health-focused products. Companies are capitalizing on regional trends by localizing offerings and investing in digital infrastructure. The competitive landscape is intensifying, with global players entering the market and local firms leveraging cultural insights and price advantages to capture market share, ensuring a vibrant, fast-paced ecosystem.
Middle East, Africa, Latin America Secondary Tickets Market Overview
The Middle East, Africa, Latin America Secondary Tickets market showcased steady development in 2024, supported by advancements in retail automation, growing awareness of sustainable practices, and increasing preference for convenience-driven solutions. Markets in the Middle East, Africa, and South America demonstrated rising adoption of digital payment solutions, recyclable packaging, and smart home innovations, catering to evolving consumer demands. From 2025, the market is poised for substantial growth, fueled by infrastructure development, the rise of digital platforms, and increasing focus on affordable, quality products. The competitive landscape is characterized by regional players innovating in product design and packaging, while international companies expand through localized strategies and strategic partnerships, ensuring that the RoW remains a critical contributor to global market dynamics.
Secondary Tickets Market Dynamics and Future Analytics
The research analyses the Secondary Tickets parent market, derived market, intermediaries’ market, raw material market, and substitute market are all evaluated to better prospect the Secondary Tickets market outlook. Geopolitical analysis, demographic analysis, and Porter’s five forces analysis are prudently assessed to estimate the best Secondary Tickets market projections.
Recent deals and developments are considered for their potential impact on Secondary Tickets's future business. Other metrics analyzed include the Threat of New Entrants, Threat of New Substitutes, Product Differentiation, Degree of Competition, Number of Suppliers, Distribution Channel, Capital Needed, Entry Barriers, Govt. Regulations, Beneficial Alternative, and Cost of Substitute in Secondary Tickets market.
Secondary Tickets trade and price analysis helps comprehend Secondary Tickets's international market scenario with top exporters/suppliers and top importers/customer information. The data and analysis assist our clients in planning procurement, identifying potential vendors/clients to associate with, understanding Secondary Tickets price trends and patterns, and exploring new Secondary Tickets sales channels. The research will be updated to the latest month to include the impact of the latest developments such as the Russia-Ukraine war on the Secondary Tickets market.
Secondary Tickets Market Structure, Competitive Intelligence and Key Winning Strategies
The report presents detailed profiles of top companies operating in the Secondary Tickets market and players serving the Secondary Tickets value chain along with their strategies for the near, medium, and long term period.
OGAnalysis’ proprietary company revenue and product analysis model unveils the Secondary Tickets market structure and competitive landscape. Company profiles of key players with a business description, product portfolio, SWOT analysis, Financial Analysis, and key strategies are covered in the report. It identifies top-performing Secondary Tickets products in global and regional markets. New Product Launches, Investment & Funding updates, Mergers & Acquisitions, Collaboration & Partnership, Awards and Agreements, Expansion, and other developments give our clients the Secondary Tickets market update to stay ahead of the competition.
Company offerings in different segments across Asia-Pacific, Europe, the Middle East, Africa, and South and Central America are presented to better understand the company strategy for the Secondary Tickets market. The competition analysis enables users to assess competitor strategies and helps align their capabilities and resources for future growth prospects to improve their market share.
Secondary Tickets Market Research Scope
• Global Secondary Tickets market size and growth projections (CAGR), 2024- 2034
• Policies of USA New President Trump, Russia-Ukraine War, Israel-Palestine, Middle East Tensions Impact on the Secondary Tickets Trade and Supply-chain
• Secondary Tickets market size, share, and outlook across 5 regions and 27 countries, 2023- 2034
• Secondary Tickets market size, CAGR, and Market Share of key products, applications, and end-user verticals, 2023- 2034
• Short and long-term Secondary Tickets market trends, drivers, restraints, and opportunities
• Porter’s Five Forces analysis, Technological developments in the Secondary Tickets market, Secondary Tickets supply chain analysis
• Secondary Tickets trade analysis, Secondary Tickets market price analysis, Secondary Tickets supply/demand
• Profiles of 5 leading companies in the industry- overview, key strategies, financials, and products
• Latest Secondary Tickets market news and developments
The Secondary Tickets Market international scenario is well established in the report with separate chapters on North America Secondary Tickets Market, Europe Secondary Tickets Market, Asia-Pacific Secondary Tickets Market, Middle East and Africa Secondary Tickets Market, and South and Central America Secondary Tickets Markets. These sections further fragment the regional Secondary Tickets market by type, application, end-user, and country.
Regional Insights
North America Secondary Tickets market data and outlook to 2034
United States
Canada
Mexico
Europe Secondary Tickets market data and outlook to 2034
Germany
United Kingdom
France
Italy
Spain
BeNeLux
Russia
Asia-Pacific Secondary Tickets market data and outlook to 2034
China
Japan
India
South Korea
Australia
Indonesia
Malaysia
Vietnam
Middle East and Africa Secondary Tickets market data and outlook to 2034
Saudi Arabia
South Africa
Iran
UAE
Egypt
South and Central America Secondary Tickets market data and outlook to 2034
Brazil
Argentina
Chile
Peru
* We can include data and analysis of additional coutries on demand
Who can benefit from this research
The research would help top management/strategy formulators/business/product development/sales managers and investors in this market in the following ways
1. The report provides 2024 Secondary Tickets market sales data at the global, regional, and key country levels with a detailed outlook to 2034 allowing companies to calculate their market share and analyze prospects, uncover new markets, and plan market entry strategy.
2. The research includes the Secondary Tickets market split into different types and applications. This segmentation helps managers plan their products and budgets based on the future growth rates of each segment
3. The Secondary Tickets market study helps stakeholders understand the breadth and stance of the market giving them information on key drivers, restraints, challenges, and growth opportunities of the market and mitigating risks
4. This report would help top management understand competition better with a detailed SWOT analysis and key strategies of their competitors, and plan their position in the business
5. The study assists investors in analyzing Secondary Tickets business prospects by region, key countries, and top companies' information to channel their investments.
Available Customizations
The standard syndicate report is designed to serve the common interests of Secondary Tickets Market players across the value chain and include selective data and analysis from entire research findings as per the scope and price of the publication.
However, to precisely match the specific research requirements of individual clients, we offer several customization options to include the data and analysis of interest in the final deliverable.
Some of the customization requests are as mentioned below –
Segmentation of choice – Our clients can seek customization to modify/add a market division for types/applications/end-uses/processes of their choice.
Secondary Tickets Pricing and Margins Across the Supply Chain, Secondary Tickets Price Analysis / International Trade Data / Import-Export Analysis,
Supply Chain Analysis, Supply – Demand Gap Analysis, PESTLE Analysis, Macro-Economic Analysis, and other Secondary Tickets market analytics
Processing and manufacturing requirements, Patent Analysis, Technology Trends, and Product Innovations
Further, the client can seek customization to break down geographies as per their requirements for specific countries/country groups such as South East Asia, Central Asia, Emerging and Developing Asia, Western Europe, Eastern Europe, Benelux, Emerging and Developing Europe, Nordic countries, North Africa, Sub-Saharan Africa, Caribbean, The Middle East and North Africa (MENA), Gulf Cooperation Council (GCC) or any other.
Capital Requirements, Income Projections, Profit Forecasts, and other parameters to prepare a detailed project report to present to Banks/Investment Agencies.
Customization of up to 10% of the content can be done without any additional charges.
Note: Latest developments will be updated in the report and delivered within 2 to 3 working days
Market Segmentation
By Ticket Type
-
Sports Events
-
Concerts & Music Festivals
-
Theatre & Performing Arts
-
Other Live Events
By Platform Type
-
Online Platforms
-
Offline Brokers
By Revenue Model
-
Commission-based Sales
-
Subscription-based Models
-
Advertising & Listing Fees
By End User
-
Individuals
-
Corporate Clients
By Geography
North America (USA, Canada, Mexico)
Europe (Germany, UK, France, Spain, Italy, Rest of Europe)
Asia-Pacific (China, India, Japan, Australia, Rest of APAC)
The Middle East and Africa (Middle East, Africa)
South and Central America (Brazil, Argentina, Rest of SCA)
List Of Companies
-
TickPick LLC
-
Ace Ticket Worldwide Inc
-
alliancetickets.com
-
Coast to Coast Tickets LLC
-
GO-tickets
-
Tickets.com Inc.
-
TicketCity Inc.
-
TiqIQ LLC
-
viagogo AG
-
Vivid Seats LLC
-
StubHub
-
Ticketmaster
1. Table of Contents
1.1 List of Tables
1.2 List of Figures
2. Global Secondary Tickets Market Review, 2024
2.1 Secondary Tickets Industry Overview
2.2 Research Methodology
3. Secondary Tickets Market Insights
3.1 Secondary Tickets Market Trends to 2034
3.2 Future Opportunities in Secondary Tickets Market
3.3 Dominant Applications of Secondary Tickets, 2024 Vs 2034
3.4 Key Types of Secondary Tickets, 2024 Vs 2034
3.5 Leading End Uses of Secondary Tickets Market, 2024 Vs 2034
3.6 High Prospect Countries for Secondary Tickets Market, 2024 Vs 2034
4. Secondary Tickets Market Trends, Drivers, and Restraints
4.1 Latest Trends and Recent Developments in Secondary Tickets Market
4.2 Key Factors Driving the Secondary Tickets Market Growth
4.2 Major Challenges to the Secondary Tickets industry, 2025- 2034
4.3 Impact of Wars and geo-political tensions on Secondary Tickets supply chain
5 Five Forces Analysis for Global Secondary Tickets Market
5.1 Secondary Tickets Industry Attractiveness Index, 2024
5.2 Secondary Tickets Market Threat of New Entrants
5.3 Secondary Tickets Market Bargaining Power of Suppliers
5.4 Secondary Tickets Market Bargaining Power of Buyers
5.5 Secondary Tickets Market Intensity of Competitive Rivalry
5.6 Secondary Tickets Market Threat of Substitutes
6. Global Secondary Tickets Market Data – Industry Size, Share, and Outlook
6.1 Secondary Tickets Market Annual Sales Outlook, 2025- 2034 ($ Million)
6.1 Global Secondary Tickets Market Annual Sales Outlook by Type, 2025- 2034 ($ Million)
6.2 Global Secondary Tickets Market Annual Sales Outlook by Application, 2025- 2034 ($ Million)
6.3 Global Secondary Tickets Market Annual Sales Outlook by End-User, 2025- 2034 ($ Million)
6.4 Global Secondary Tickets Market Annual Sales Outlook by Region, 2025- 2034 ($ Million)
7. Asia Pacific Secondary Tickets Industry Statistics – Market Size, Share, Competition and Outlook
7.1 Asia Pacific Market Insights, 2024
7.2 Asia Pacific Secondary Tickets Market Revenue Forecast by Type, 2025- 2034 (USD Million)
7.3 Asia Pacific Secondary Tickets Market Revenue Forecast by Application, 2025- 2034(USD Million)
7.4 Asia Pacific Secondary Tickets Market Revenue Forecast by End-User, 2025- 2034 (USD Million)
7.5 Asia Pacific Secondary Tickets Market Revenue Forecast by Country, 2025- 2034 (USD Million)
7.5.1 China Secondary Tickets Analysis and Forecast to 2034
7.5.2 Japan Secondary Tickets Analysis and Forecast to 2034
7.5.3 India Secondary Tickets Analysis and Forecast to 2034
7.5.4 South Korea Secondary Tickets Analysis and Forecast to 2034
7.5.5 Australia Secondary Tickets Analysis and Forecast to 2034
7.5.6 Indonesia Secondary Tickets Analysis and Forecast to 2034
7.5.7 Malaysia Secondary Tickets Analysis and Forecast to 2034
7.5.8 Vietnam Secondary Tickets Analysis and Forecast to 2034
7.6 Leading Companies in Asia Pacific Secondary Tickets Industry
8. Europe Secondary Tickets Market Historical Trends, Outlook, and Business Prospects
8.1 Europe Key Findings, 2024
8.2 Europe Secondary Tickets Market Size and Percentage Breakdown by Type, 2025- 2034 (USD Million)
8.3 Europe Secondary Tickets Market Size and Percentage Breakdown by Application, 2025- 2034 (USD Million)
8.4 Europe Secondary Tickets Market Size and Percentage Breakdown by End-User, 2025- 2034 (USD Million)
8.5 Europe Secondary Tickets Market Size and Percentage Breakdown by Country, 2025- 2034 (USD Million)
8.5.1 2024 Germany Secondary Tickets Market Size and Outlook to 2034
8.5.2 2024 United Kingdom Secondary Tickets Market Size and Outlook to 2034
8.5.3 2024 France Secondary Tickets Market Size and Outlook to 2034
8.5.4 2024 Italy Secondary Tickets Market Size and Outlook to 2034
8.5.5 2024 Spain Secondary Tickets Market Size and Outlook to 2034
8.5.6 2024 BeNeLux Secondary Tickets Market Size and Outlook to 2034
8.5.7 2024 Russia Secondary Tickets Market Size and Outlook to 2034
8.6 Leading Companies in Europe Secondary Tickets Industry
9. North America Secondary Tickets Market Trends, Outlook, and Growth Prospects
9.1 North America Snapshot, 2024
9.2 North America Secondary Tickets Market Analysis and Outlook by Type, 2025- 2034($ Million)
9.3 North America Secondary Tickets Market Analysis and Outlook by Application, 2025- 2034($ Million)
9.4 North America Secondary Tickets Market Analysis and Outlook by End-User, 2025- 2034($ Million)
9.5 North America Secondary Tickets Market Analysis and Outlook by Country, 2025- 2034($ Million)
9.5.1 United States Secondary Tickets Market Analysis and Outlook
9.5.2 Canada Secondary Tickets Market Analysis and Outlook
9.5.3 Mexico Secondary Tickets Market Analysis and Outlook
9.6 Leading Companies in North America Secondary Tickets Business
10. Latin America Secondary Tickets Market Drivers, Challenges, and Growth Prospects
10.1 Latin America Snapshot, 2024
10.2 Latin America Secondary Tickets Market Future by Type, 2025- 2034($ Million)
10.3 Latin America Secondary Tickets Market Future by Application, 2025- 2034($ Million)
10.4 Latin America Secondary Tickets Market Future by End-User, 2025- 2034($ Million)
10.5 Latin America Secondary Tickets Market Future by Country, 2025- 2034($ Million)
10.5.1 Brazil Secondary Tickets Market Analysis and Outlook to 2034
10.5.2 Argentina Secondary Tickets Market Analysis and Outlook to 2034
10.5.3 Chile Secondary Tickets Market Analysis and Outlook to 2034
10.6 Leading Companies in Latin America Secondary Tickets Industry
11. Middle East Africa Secondary Tickets Market Outlook and Growth Prospects
11.1 Middle East Africa Overview, 2024
11.2 Middle East Africa Secondary Tickets Market Statistics by Type, 2025- 2034 (USD Million)
11.3 Middle East Africa Secondary Tickets Market Statistics by Application, 2025- 2034 (USD Million)
11.4 Middle East Africa Secondary Tickets Market Statistics by End-User, 2025- 2034 (USD Million)
11.5 Middle East Africa Secondary Tickets Market Statistics by Country, 2025- 2034 (USD Million)
11.5.1 South Africa Secondary Tickets Market Outlook
11.5.2 Egypt Secondary Tickets Market Outlook
11.5.3 Saudi Arabia Secondary Tickets Market Outlook
11.5.4 Iran Secondary Tickets Market Outlook
11.5.5 UAE Secondary Tickets Market Outlook
11.6 Leading Companies in Middle East Africa Secondary Tickets Business
12. Secondary Tickets Market Structure and Competitive Landscape
12.1 Key Companies in Secondary Tickets Business
12.2 Secondary Tickets Key Player Benchmarking
12.3 Secondary Tickets Product Portfolio
12.4 Financial Analysis
12.5 SWOT and Financial Analysis Review
14. Latest News, Deals, and Developments in Secondary Tickets Market
14.1 Secondary Tickets trade export, import value and price analysis
15 Appendix
15.1 Publisher Expertise
15.2 Secondary Tickets Industry Report Sources and Methodology
Research Methodology
Our research methodology combines primary and secondary research techniques to ensure comprehensive market analysis.
Primary Research
We conduct extensive interviews with industry experts, key opinion leaders, and market participants to gather first-hand insights.
Secondary Research
Our team analyzes published reports, company websites, financial statements, and industry databases to validate our findings.
Data Analysis
We employ advanced analytical tools and statistical methods to process and interpret market data accurately.
Get Free Sample
At OG Analysis, we understand the importance of informed decision-making in today's dynamic business landscape. To help you experience the depth and quality of our market research reports, we offer complimentary samples tailored to your specific needs.
Start Now! Please fill the form below for your free sample.
Why Request a Free Sample?
Evaluate Our Expertise: Our reports are crafted by industry experts and seasoned analysts. Requesting a sample allows you to assess the depth of research and the caliber of insights we provide.
Tailored to Your Needs: Let us know your industry, market segment, or specific topic of interest. Our free samples are customized to ensure relevance to your business objectives.
Witness Actionable Insights: See firsthand how our reports go beyond data, offering actionable insights and strategic recommendations that can drive your business forward.
Embark on your journey towards strategic decision-making by requesting a free sample from OG Analysis. Experience the caliber of insights that can transform the way you approach your business challenges.
FAQ's
The Global Secondary Tickets Market is estimated to generate USD 3.4 Billion in revenue in 2025
The Global Secondary Tickets Market is expected to grow at a Compound Annual Growth Rate (CAGR) of 9.2% during the forecast period from 2025 to 2034.
The Secondary Tickets Market is estimated to reach USD 7.8 Billion by 2034.
$3950- 30%
$5850- 40%
$7850- 50%
$2850- 20%
Didn’t find what you’re looking for? TALK TO OUR ANALYST TEAM
Need something within your budget? NO WORRIES! WE GOT YOU COVERED!